Why is Ice Less Dense Than Water
This reduces the density of ice because the ice has more space between the molecules. Why is ice less dense than water.
In the first ice crystal there are spaces between some of the molecules which is not there in the second crystal structure.

. The water molecules form crystalline lattice structures in ice where they are more spread out d. What happens to water under ice. Ice floats because it is about 9 less dense than liquid water.
The water molecules in ice have less volume than in liquid ice. B Some water molecules are lost when water freezes. This is what happens when enough thermal energy is present to break the rigid hydrogen bonds resulting in melting.
Yes some ice is denser than water. As a solid ice is denser than water but will still float due to the high surface tension of water When water freezes the molecules arrange into a crystal form in which they are farther apart than in liquid water As water freezes it forms cubic crystals made only of eight atoms. Water is considerably less dense than its solid counterpart ice and this unusual property is considered to be due to the hydrogen bonding in water.
Below 4 Celsius water becomes less dense as it gets colder causing water about to freeze to float to the top. In other words ice takes up about 9 more space than water so a liter of ice weighs less than a liter water. Clearly once this crystaline structure is no longer forced into place by the rigid hydrogen bonding in ice it can collapse into itself resulting a greater density of water molecules.
In order for ice to be less dense than water the molecules in ice are more organized. How bout this beastly answer. The heavier water displaces the lighter ice so ice floats to the top.
Together they are attached by a very strong bond called a covalent bond. Why is ice less dense than water. Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart which lowers the density.
10 Why Is Ice Less Dense Than Liquid Water Quizlet. The water molecules are more closely packed together in ice than in water. Why is ice less dense than water.
Why ice is floating on water. Answer 1 of 16. Public Domain Image source.
This is because the hydrogen bonds in water are randomly orientated which means that the molecules are closer together. Ice density waterWhy is the density of ice less than than the density of water why ice floats in water the density of ice and the density of water. As it cools further and freezes into ice it actually becomes less dense.
If you put pressure on regular ice and give it time to rearrange the molecules will move into a new crystal lattice which results in the ice being more dense than water. When water freezes water molecules form a crystalline structure maintained by hydrogen bonding. Therefore ice is less dense than water.
The bond between the different water molecules is called a hydrogen bond this one is a little weaker. In ice the hydrogen bonds hold the water molecules apart in an open lattice structure. Yes which is precisely why ice frozen water floats in liquid water.
The water molecules in ice have less mass than in liquid water b. Why is ice less dense than water. Answer 1 of 5.
The reason why ice is less dense than water is simple. Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart which lowers the density. A Water molecules slow their vibrations and move closer together when water freezes.
Thus the liquid form of water although. Ice is less dense than water because the particles of the solid ice is more spread out while the water particles are as close as they. When water freezes water molecules form a crystalline structure maintained by hydrogen bonding.
Solid water or ice is less dense than liquid water. C Ice crystallizes with an open structure and the gaps that form between the water molecules in ice increase its volume. As you know two atoms of hydrogen and one atom of oxygen make water.
Right when the water freezes to ice the ice becomes significantly less dense than the water and continues to float on the lakes surface. Why is ice less dense than water intermolecular forces.
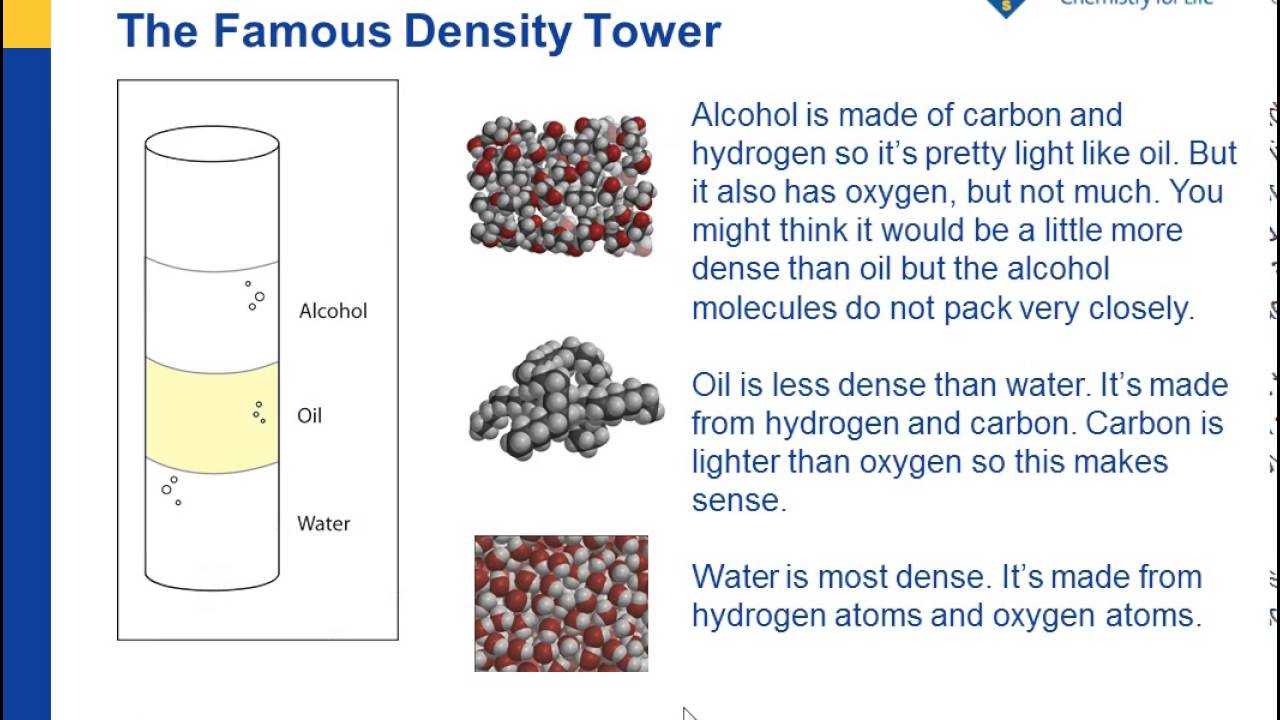
Density Sink And Float For Liquids Chapter 3 Density Middle School Chemistry Middle School Chemistry Teaching Chemistry Chemistry

Ice Floats Because Solid Water Is Less Dense Than Liquid Water Arctic Landscape Landscape Outdoor

Because Of Hydrogen Bonding Water Is Less Dense As A Solid Than It Is As A Liquid Consequently Ice Floats Language Chemistry Education

Scientific Explanation Of Why Ice Floats Elementary Science Hydrogen Bond Floating

No comments for "Why is Ice Less Dense Than Water"
Post a Comment